On two new grants, the Van Nostrand lab is developing the field’s first gene-edited CAA rat models to help investigate the role of brain blood vessels in disorders related to Alzheimer’s disease.
January 27, 2023 | Blood vessels in the brain likely hold important clues as to how and why Alzheimer’s disease develops—yet existing models are limited in what they can show us about the role the cerebral vasculature is playing in the disease. As one of the field’s foremost experts in small vessel diseases in the brain, William Van Nostrand understands that a better model would open new possibilities for researchers to answer critical questions.
This past fall, his lab began work on two new NIH grants to develop gene-edited rat models for cerebral small vessel diseases, including cerebral amyloid angiopathy (CAA), a condition that causes symptoms such as dementia and is linked to Alzheimer’s disease. The models will be the first of their kind for the field.
“A model is only as good as how well it reflects the disease you are studying,” says Van Nostrand, Hermann Professor of Neuroscience and Co-Executive Director of the George & Anne Ryan Institute for Neuroscience. “As we have made advances in our understanding of Alzheimer’s disease and related disorders, we needed to create models that are more closely aligned with pathology in humans.”
Transgenic rodent models—in which genetic material is artificially inserted into a mouse or rat embryo—are the most common models to study brain diseases, but they offer an incomplete picture. “Transgenic models are extremely useful for studying certain aspects of the disease, but they don’t provide an accurate representation of how the disease develops and progresses,” says Van Nostrand. “In a transgenic model, you are introducing a foreign gene that typically gets expressed from only one type of cell, typically a neuronal cell, and not accounting for contributions from other sources, such as glial cells and blood vessels.”
Despite decades of research, the cause of Alzheimer’s disease remains unknown. Researchers have identified potential pieces in the puzzle — such as the build-up of amyloid-beta protein “plaques” and tau protein “tangles” found in the brains of people with Alzheimer’s disease — but it is still unclear why this toxic build-up occurs, or even if plaques and tangles are primary causes of the disease.
“This gives us a model that is more reflective of what is happening in the human brain during disease.”William Van Nostrand, Herrmann Professor of Neuroscience and Co-Executive Director of the George & Anne Ryan Institute for Neuroscience
Meanwhile, recently FDA-approved Alzheimer’s drugs remove this amyloid build-up but have only shown a very modest impact on cognitive decline. “My belief is that amyloid is an important trigger but not necessarily a determinant of dementia,” says Van Nostrand. “We know that amyloid sets off inflammation and a pathological cascade, but it could be another one of those inflammatory factors, or a combination, that drives the disease.”
Van Nostrand’s CAA gene-edited rat model will provide a more “natural” representation of how amyloid deposits accumulate in the brain blood vessels. “We know that a mutation can cause the amyloid beta protein to begin accumulating in the blood vessels, so when we create the same mutation in rats, they produce similar vascular amyloid deposits in a similar way,” he says. “This gives us a model that is more reflective of what is happening in the human brain during the disease.”
The gene-edited CAA rat model will not only provide a platform to investigate inflammation and vessel damage, but also to help understand critical lifestyle factors in Alzheimer’ disease development and progression.
Last spring, Van Nostrand and researchers at Yale University published compelling findings on the role of clearance in the brain, showing that a breakdown in brain “waste management” systems contributes to the build of amyloid and neurotoxins. Notably, lifestyle factors, including sleep and physical activity, play a key role in the healthy function of this process.
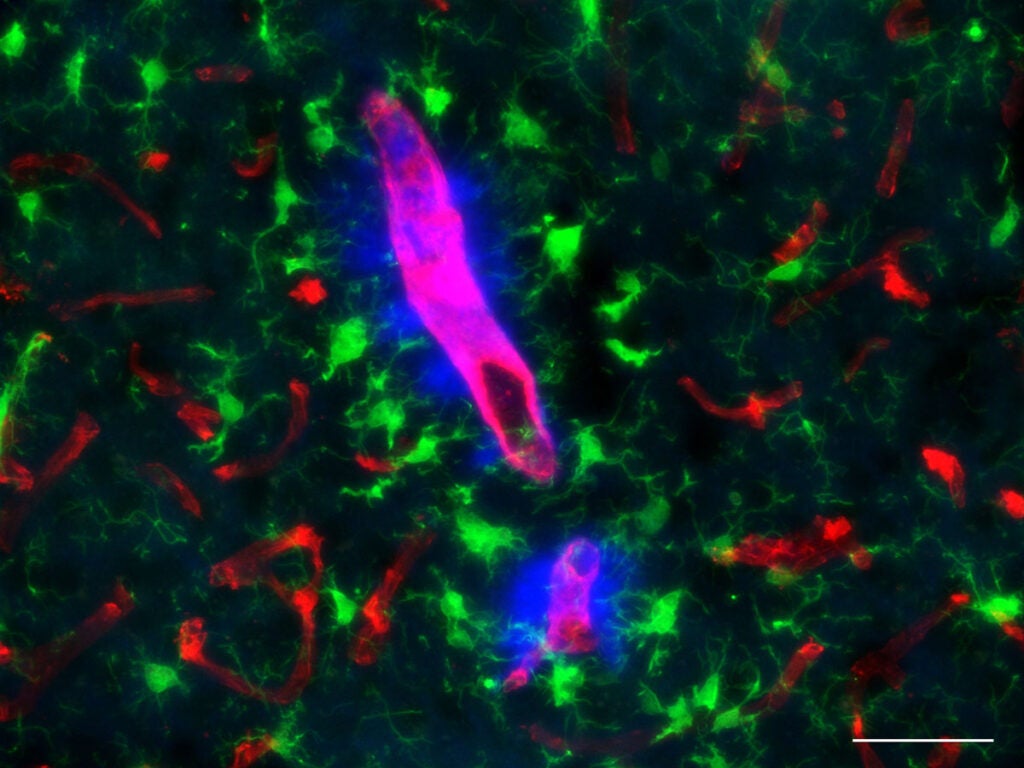
With the new models, Van Nostrand and other researchers can explore more deeply these factors, as well as help identify biomarkers and potential therapeutic interventions that can stop and slow disease progression.
“We have learned a lot so far, but we still do not know enough to prevent and cure the disease. The more we learn mechanistically, the more we have an opportunity to intervene or have an ability to test something that could be effective at the earlier stages of disease.”
This isn’t the first time Van Nostrand has helped to advance the field in creating better ways to study disease. His research career began at UC Irvine, where he worked on in vitro systems, culturing cells from human blood vessels, when he realized that he couldn’t study all the questions he wanted to ask. “We started hitting a wall in terms of what we were able to learn,” he said. He subsequently developed a transgenic mouse model that helped open new possibilities for his own research and that of others. He hopes that the gene-edited rats will have similar benefits for the field.
Van Nostrand says, “With a more biologically relevant model, we can investigate important questions that we have not been able to study before.”
