IN THE LAB
Finding the Right Fit
JUNE 2018
Can you create a thrombin-inhibiting drug that doesn’t totally inhibit thrombin? Graduate student Shelby Johnson is working to solve the puzzle.
WHEN BLOOD FORMS a clot, the protein thrombin plays a key role in the coagulation process. A doctor might therefore prescribe a thrombin-inhibiting drug as a blood-thinner for patients at risk of stroke or deadly clots.
But Shelby Johnson is trying to figure out how to create a thrombin inhibitor that keeps thrombin’s role in clotting.
A graduate student in neuroscience, Johnson has been working with the lab of Paula Grammas, executive director of the Ryan Institute, to look at the potential of thrombin inhibitors in treating Alzheimer’s disease. Based on Grammas’s decades of research investigating the role of blood vessels in the development and progression of Alzheimer’s, the Grammas lab has identified thrombin as one of the key drivers in a dysfunctional cycle where injured blood vessels—as a result of an event such as stroke or daily stressors like high blood pressure, high cholesterol, or high blood sugar—begin producing toxins that contribute to the brain cell death associated with Alzheimer’s. Inhibiting thrombin, Grammas has hypothesized, could help slow or stop this cycle.
While Grammas will soon test this hypothesis in a Phase I clinical trial, Johnson has been at work to figure out how a thrombin inhibitor could be used to block thrombin’s role in the toxic cycle without interrupting its important function in coagulation.
“It’s definitely a puzzle,” Johnson says. “But I like the challenge of solving a problem that could be really impactful.”
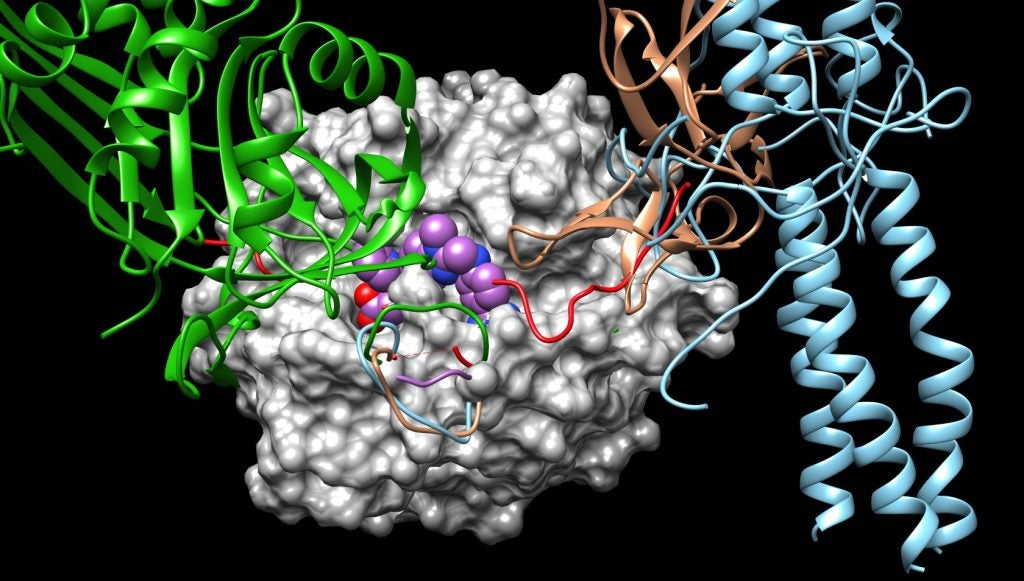
Thrombin converts fibrinogen, a soluble protein that circulates in the blood, to the insoluble fibrin that forms a blood clot at the site of injury. In the process, thrombin also activates receptors on the cells lining the blood vessels, promoting an inflammatory response. Grammas’s research shows that in Alzheimer’s disease, thrombin is driving over-inflammation that triggers the production of more thrombin and more toxic proteins that kill neurons.
Using state-of-the-art computer modeling, Johnson has worked to identify potential compounds that can block the site where the receptors typically interact with thrombin in order to stop the inflammation cycle. She is also looking to see whether inhibiting thrombin in this way leads to unintended changes to the protein that could affect its other interactions.
“This is where our focus will be going forward,” says Johnson.
Once she has identified promising drug candidates, Johnson will work with Brenton DeBoef, a professor of chemistry at URI, to create a synthetic thrombin-inhibiting drug that could ultimately be tested in the Grammas lab’s research.
It’s something of a detour from where she started, as a biology and chemistry undergraduate who began working in the natural products lab of Associate Professor of Biomedical and Pharmaceutical Sciences Navindra Seeram, also a Ryan Research Associate Professor of Neuroscience, as part of a RI-INBRE (Rhode Island – IDeA Network of Biomedical Research Excellence) research program. But the challenges and complexities of drug design, combined with the rewards of working in an area of research that has touched her personally (she has Parkinson’s disease in her family) have motivated her.
“If it doesn’t work, we’ll go back and try again,” Johnson says. “Even when things don’t work, you can stumble onto something you didn’t expect. There is always something to be learned.”
– Nicole Maranhas
